News Story
Wachsman lab develops highest oxygen-ion conductivity material
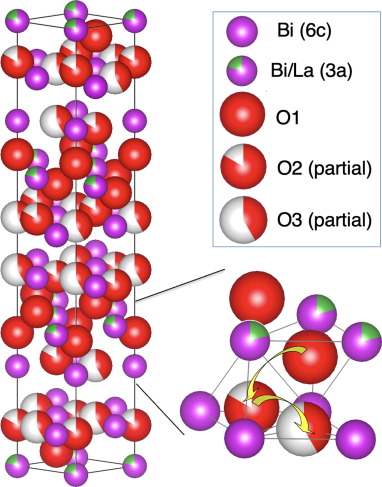
Transport of oxygen ions in rhombohedral Bi2O3 with 3D diffusion network in the ab plane and across the ab plane. Oxygen diffusion pathways are shown in yellow arrows.
Oxygen is a vital molecule for sustaining aerobic life and plays a crucial role across various industries, including biomedical, semiconductor, chemical, transportation, and energy. To enhance the efficiency and practicality of solid-state oxygen-ion conducting technologies, high-conductivity electrolytes are essential, as they help lower operating temperatures. This advancement supports improved performance in a range of applications, from oxygen generation to energy conversion and storage.
Dr. Eric Wachsman, University of Maryland (UMD) Distinguished University Professor and Director of the Maryland Energy Innovation Institute (MEI2) just published a paper in Materials Today detailing his lab’s development of bismuth oxide electrolytes designed to operate effectively at high and low temperature ranges. At elevated temperatures (≥600 °C), they demonstrated a cubic phase of Bi₂O₃ exhibiting the highest oxygen-ion conductivity ever recorded for a phase-stable electrolyte, maintaining performance over 100 hours at 650 °C. For lower temperatures (<600 °C), a rhombohedral Bi₂O₃ phase was developed, offering remarkable stability with no detectable decline in conductivity after 100 hours of aging at 500 °C. This makes it the most efficient and stable oxygen-ion conducting electrolyte for lower-temperature solid oxide cells (SOCs).
This work is the culmination of 35 years of research developing a double-doping approach to increase both the conductivity and stability of solid-state oxide-ion conductors. The breakthrough will lead to significantly improved performance in high-temperature devices like solid oxide fuel cells (SOFCs), oxygen sensors, and oxygen separation membranes. Additionally, operating costs will be reduced as operating at lower temperatures reduces the need for expensive high-temperature materials and insulation, lowering system costs and improving safety. Improved conductivity and stability also mean better energy conversion efficiency and lower emissions, especially in fuel cell and energy storage technologies.
“Bismuth oxides were first discovered as fast oxide-ion conductors back when I was a graduate student, however, they had stability issues that limited their adoption. As a student I discovered that the conducting structure was not stable resulting in conductivity decay and then spent three decades since to understand how the local lattice structure influenced conductivity and stability, ultimately developing an approach of using two different radii cations to stabilize the highly conductive structures. With these new stable compositions bismuth oxides can finally deliver on their promise for a wide variety of oxygen production and use applications” said Wachsman.
For more information the full article can be read here:
Adam G. Jolley, Qiang Bai, Rishvi Jayathilake, Yifei Mo, Eric D. Wachsman, Bismuth oxide electrolytes with superior conductivity and stability, Materials Today, 2025, ISSN 1369-7021, https://doi.org/10.1016/j.mattod.2025.03.030
Published April 17, 2025









