Assistant Professor Joonil Seog
Self-assembly is one of the most important mechanisms that nature exploits to build a complex structure. Numerous research groups are focusing on controlling the self-assembling process to develop nanostructure of periodic patterns. The spontaneous organization often occurs in a hierarchical manner in a wide range of length scales, creating a higher-order structure which is built from a pre-assembled structure in a stepwise fashion.
During this process, the preformed substrate often provides crucial cues in facilitating a subsequent assembly step. These cues are composed of local geometric constraint and mostly non-covalent interactions such as hydrogen bonding, hydrophobic or electrostatic interactions. The formation of 1-dimensional peptide based self-assembled nanostructures on the substrate has been reported. However, in these cases controlling location and direction of the nanostructures on the substrate to create a well-defined 1-dimensional nanoscale pattern has been quite challenging.
Here, we report on applying nanomechanical forces in a local manner to initiate the self-assembly of peptide-based nanofibers at specific locations with control over their growth direction without using any local geometric constraint, such as a preformed surface pattern. This method may provide a simple way to prepare a well-ordered nanofiber substrate which can serve as a building template for functional nanoscale devices.
Self-assembling peptides have received a significant attention from nanotechnology for bottom-up fabrication and biomimetic structure. The silk-elastin like peptide polymer (SELP) is a genetically engineered block copolymer made of silk-like blocks (Gly-Ala-Gly-Ala-Gly-Ser) from Bombyx mori (silkworm) and elastin-like blocks (Gly-Val-Gly-Val-Pro) from mammalian elastin. The repeating unit of the SELP-815K contains eight silk (S) and fifteen elastin (E) units and one lysine (K) modified elastin.
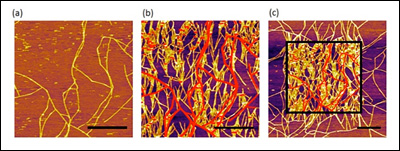
[Fig. 1. AFM images of SELP-815K nanofibers obtained in a tapping mode. (a) The first scanned image, (b) the second scanned image of the same area, (c) the zoomed out image that includes the second scanned area. The scale bars correspond to 1 μm and the red lines are original fibers and at same height scale.]
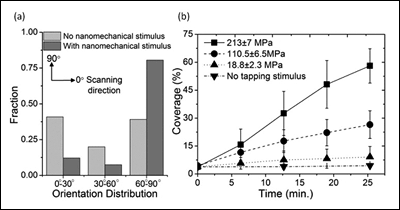
[Fig. 2. (a) Histogram of nanofiber orientation distribution with and without nanomechanical stimulus by AFM, (b) Percent increase of nanofiber coverage measured under various tapping pressures as a function of time (n=5). The coverages under a specific pressure were obtained by imaging the same area repeatedly at different time points. The coverages with no tapping stimulus were obtained from first scanned images at specific time points.]
Figure 1a is the first scanned image using atomic force microscopy (AFM) which shows some nanofibers formed on a mica substrate during incubation. When the same area was scanned again after 6 minutes, the density of nanofibers significantly increased (Figure 1b). The red lines in Figure 1b represent the nanofibers imaged in the first scan. The drastic effect of the AFM scan on nanofiber growth is clearly visible in the zoomed out image in Figure 1c, where a sharp contrast in nanofiber density was clearly observed between the area scanned once (outside the square) and the area scanned twice (inside the square). This remarkable difference clearly indicates the strong effect of the AFM tapping force on the self-assembly of nanofibers. Furthermore, the newly grown nanofibers, as a result of AFM scanning, seemed to have a strong preference in their growth direction. We used the ImageJ software to analyze the orientation of each nanofiber with respect to the AFM scanning direction (horizontal in the AFM images), shown in Figure 2a (above right). The orientation of the nanofibers formed during incubation (without any mechanical stimulus) was evenly distributed between 0° and 90°, indicating that the crystal plane of the mica had no effect on their growth direction. In sharp contrast, 80% of the newly grown fibers after nanomechanical stimulus by AFM showed preferred orientation between 60° and 90°. This suggests preferential growth perpendicular to the AFM scanning direction, and confirms the effect of tapping mode imaging on self-assembly.
To investigate the novel effect of nanomechanical stimulus on self-assembly further, the ratio of the amplitude setpoint and the free amplitude in tapping mode AFM were varied. In this mode of imaging, the AFM tip intermittently contacts the surface, exerting a momentary impact. By varying the setpoint amplitude and the free amplitude, the tapping force level was adjusted systematically. To quantify the tapping force exerted on the surface, the tapping mode imaging process was simulated using a 2-eigenmode cantilever model. The calculated force level was normalized by the nominal radius of the AFM tip to obtain an approximate pressure in each condition. The coverage of nanofibers under calculated tapping pressures varying from 18.8 MPa to 213 MPa was then measured at five different time points (Figure 2b). Overall, coverage increased as a function of time under the range of pressures tested. The rate of percent coverage change was increased from ~ 0.2 %/min. at 18.8 MPa to ~2 %/min. at 213 MPa. This observation clearly highlights the effect of local nanomechanical pressure on enhancing the self-assembly of SELP-815K nanofibers.
In conclusion, we demonstrated that the local tapping pressure exerted by AFM tip can initiate and promote the peptide self-assembly on mica surface in a force dependent manner. The growth direction of the nanofiber was largely perpendicular to scanning direction, indicating that this novel method can be utilized to create a 1-dimensional nanostructure patterned surface using nanomechanical stimulus.
References:
W. Hwang, B. Kim, R. Dandu, J. Cappello, H. Ghandehari, and J. Seog. “Surface induced nanofiber growth by self-assembly of a silk-elastin-like protein polymer”, Langmuir, 2009, 25(21); 12682-12686.
J. Chang, Xiu-Feng, Peng, K. Hijji, J. Cappello, H. Ghandehari, S. D. Solares, and J. Seog. “Nanomechanical Stimulus Accelerates and Directs the Self-Assembly of Silk-Elastin-like Nanofibers” Journal of the American Chemical Society Communications, 2011, 133(6); 1745-1747.
Top