Adjunct Professor John J. Rush
Dr. Hui Wu
Dr. Yun Liu
Auto manufacturers (GM, Toyota, etc) are investing billions of dollars in research and development of fuel-cell based cars and trucks, which require major advances in the production and storage of hydrogen to greatly reduce CO2 emission and allow a transition away from the use of the world's diminishing oil supplies. One of the major challenges in the quest to develop hydrogen-fueled vehicles is the development of light-weight materials that will absorb and release hydrogen at moderate temperatures and pressures. The Energy Department has set aggressive goals and timelines for such materials, aiming for 6% by weight by 2010 and 9% over the next decade.
MSE researchers Dr. Yun Liu, Dr. Hui Wu, and Adjunct Professor John J. Rush have been engaged in joint research with scientists at the NIST Center for Neutron Research (NCNR) investigating the key properties of metal hydrides and new coordination framework compounds. This work is one of many research efforts carried out under the University of Maryland/NIST cooperative research program on Neutron Measurements and Science managed by MSE Professor and Chair Robert M. Briber.
This cooperative research makes use of five neutron research instruments at the NCNR (three of which were developed jointly by Maryland and NIST scientists and engineers) to probe the atomic and molecular structure and dynamical behavior of hydrogen bound in storage materials. Neutrons are uniquely sensitive to hydrogen (10-20 times other elements), and these instruments are currently the best collection of such instruments in the United States.
Recent research accomplishments by the Maryland/NIST team include the determination of the detailed location and rotational behavior of hydrogen and methane in new metal organic framework materials (MOFs). The arrangement of hydrogen molecules absorbed in one of these MOFs is shown in figure 1 below. The range of H2 bonding sites and strengths along with rotational potentials are a key to H2 storage capacity and release in these materials.
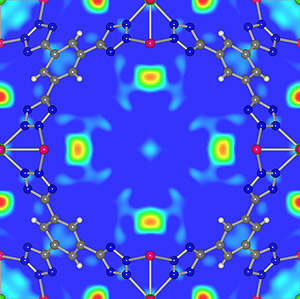
Figure 1. Hydrogen molecules adsorption sites (red-yellow regions) in Mn-BTT MOF.1
MSE scientists are also leading the way in the study of new light weight metal hydrides, aimed at finding new compounds and paths leading to hydride destabilization for hydrogen absorption and release at lower temperatures. Recent accomplishments include the discovery of previously unknown ternary hydride phases in lithium-silicon (germanium) alloys and the improvement of hydrogen-storage performance by amorphizatio (see figure 2 below).
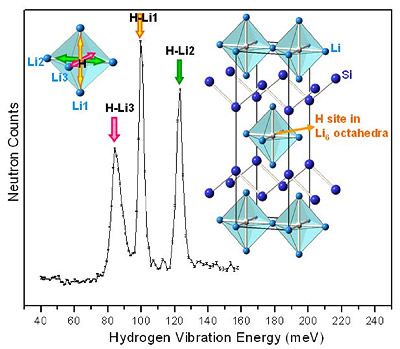
Figure 2. Structure & vibration of hydrogen atoms bound in Li4Si2H.2,3
The Maryland/NIST hydrogen storage research is being presented in a number of invited lectures, including national meetings of the American Physical Society, Materials Research Society and the American Nuclear Society. A poster presented by Hui Wu on this research was awarded top prize for materials research at the annual Sigma Xi poster competition at NIST. The Maryland/NIST team has also formed collaborations with colleagues across the U.S., including CalTech, the Jet Propulsion Laboratory, Sandia, HRL Laboratories, the National Renewable Energy Laboratory, UC Berkeley, and the University of Pennsylvania. Over fifteen publications have resulted from this research effort in the past two years.
References:
[1] Dinca, M.; Dailly, A.; Liu, Y.; Brown, C. M.; Neumann, D. A.; Long, J. R.; Hydrogen Storage in a Microporous Metal-Organic Framework with Exposed Mn2+ Coordination Sites, J. Am. Chem. Soc.128, 16876-16883 (2006).
[2] Wu, H.; Hartman, M. R.; Udovic, T. J.; Rush, J. J.; Zhou, W.; Bowman, Jr., R.C.; Vajo, J. J. "Crystal Structure of the Novel Ternary Hydrides Li4Tt2D (Tt=Si and Ge)", Acta. Cryst. B63, 63-68 (2007)
[3] Wu, H.; Zhou, W.; Udovic, T. J.; Rush, J. J.; Yildirim, T.; Hartman, M. R.; Bowman, Jr., R.C.; Vajo, J. J. "Neutron vibrational spectroscopy and first-principles calculations of the ternary hydrides Li4Si2H(D) and Li4Ge2H(D): Electronic structure and lattice dynamics," Phys. Rev. B, submitted
Top