Xiaojun Kuang1
Mark A. Green2,3
Hongjun Niu1
Pawel Zajdel2
Calum Dickinson1
John B. Claridge1
Laurent Jantsky1
Matthew J. Rosseinsky1
1 Department of Chemistry, The University of Liverpool, Liverpool, UK
2 NIST Center for Neutron Research, NIST, Gaithersburg, Md. USA
3 Department of Material Science and Engineering, University of Maryland
Fuel cells date back to the mid-19th century, when the concept was first proposed by the Swiss chemist Schonbein. They have been employed in numerous applications through the years, including providing both electricity and drinking water in NASA space programs. The principles underpinning fuel cells rely on the actual generation of electricity within the cell, as opposed to a battery that provides stored energy.
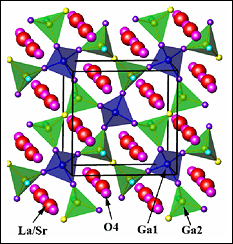
[Figure 1: Structure of La1.54Sr0.46Ga3O7.27 which stablises in the Mellite structure. The O4 positions indicate additional interstitial oxygen ions, not found in the parent structure.]
The Solid Oxide Fuel Cell (SOFC) is a particularly promising variety of cell and is characterized by very favorable power output but at the cost of high operating temperatures (» 850° C). The electricity in these cells is produced from the electrochemical reaction between a fuel, such as hydrogen, on the anode side and oxygen on the cathode side. The reaction product of simply water (or steam) has no environmental implications.
Although fuels cells have greatly improved since the early alkali-based cells used in the Apollo space program, they are still not competitive on cost or power output with the ubiquitous internal combustion engine. The development of more efficient systems requires the identification of new materials.
SOFCs are entirely composed of ceramic material and a high operating temperature is a prerequisite to generating sufficient oxygen mobility. The time presently taken to heat typical commercial fuel cells to their operating temperature is anywhere from 45 minutes to several hours. This hinders their use in many applications such as a primary energy source in automobiles, where the near ambient temperature proton exchange membrane design of fuel cell is more viable. Though, applications have been identified such as stationary power generators on the megawatt scale and mobile ancillary power sources for refrigeration.
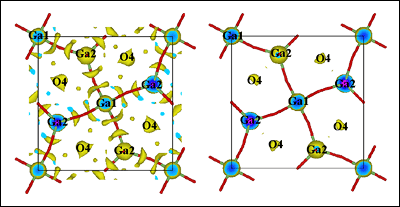
[Figure 2: Comparison of the nuclear scattering density of La1.54Sr0.46Ga3O7.27 as obtained from maximum entropy analysis maps at ambient (left) and (right) 800° C]
Oxide mobility in solids is associated with defects. Typical fuel cells use vacancies such as in yttria-stabilized zirconia oxide, which delivers the necessary high oxygen ion conductivity along with low electrical conductivity. Materials where excess (interstitial) oxide anions are the source of oxygen mobility are much less studied. A group of scientists from University of Maryland, NIST and University of Liverpool have recently used neutron diffraction to study a new system they identified to have interstitial oxide ions, shown in Figure 1. It is composed of Gallium oxide polyhedron and they discovered that it delivers comparable performance to the systems containing vacancies, but at much lower temperatures.
The team has used an image reconstruction technique called maximum entropy, more at home with radio astronomers, to extract as much information from their neutron data as possible. The technique has revealed a sophisticated relaxation mechanism. This relaxation process explains the ability of the melilite structure to accommodate the interstitial oxygen ion via local lattice relaxation as well as and sustain oxygen mobility. The understanding of this process opens up many new structural families of materials as candidates for high performance interstitial oxide conductors.
References
[1] X. Kuang, M. A. Green, H. Hiu, P. Zajdel, C. Dickerson, J. B. Claridge, L. Jantsky and M. J. Rosseinsky, Nature Materials 7, 498 (2008).
Top