Press Release
High Temperature Thermal Shocks Increase Stability of Single Atom Catalysts
Multi-institutional study published in Nature Nanotechnology.
FOR IMMEDIATE RELEASE August 12, 2019
CONTACT:
Katie Doyle
301 405 0379
khollan3@umd.edu
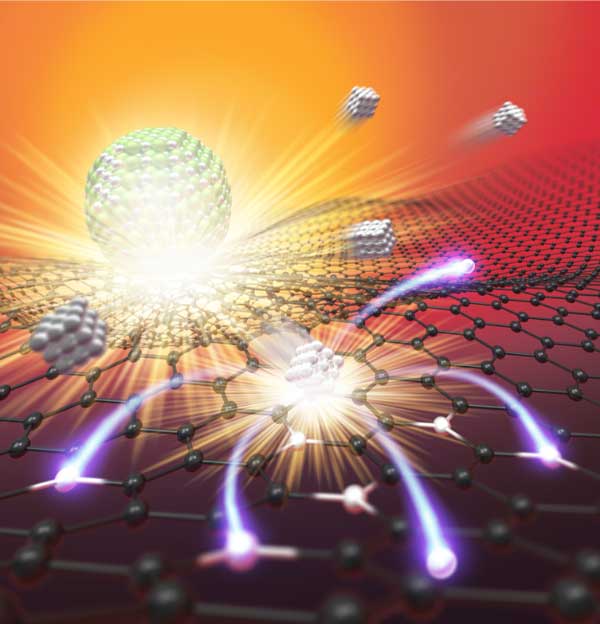
Catalysts are essentially boosters that increase the rate of a chemical reaction, and are widely used in the fields of petroleum refining, coal and natural gas conversion, and ammonia production, to name a few. Catalysts also drive emerging battery and fuel cell technology, which is usually (thermally or electrically) energy-intensive, thus requiring catalysis to reduce the reaction temperature, pressure, or electrochemical over-potentials.
Single atom catalysts maximize the metal utilization efficiency of each atom and provide superior performance, representing the frontier of catalysis. Single atoms, however, are typically unstable when synthesized at low temperature (e.g., less than 1000K), and tend to re-aggregate into nanoparticles as a means of minimizing surface energy. To that end, a research team in the University of Maryland (UMD) Department of Materials Science and Engineering (MSE) developed a high temperature shockwave catalysis method - reaching up to 3000K, which is half the temperature of the sun - intended to "anchor" single atoms onto the substrate, offering superior thermal stability.
The research team led by MSE Professor Liangbing Hu, published their study in Nature Nanotechnology on August 12. Yonggang Yao, MSE Ph.D. Student and member of Dr. Hu's research team, served as the lead author on the paper.
"Our method is achieved using periodic on-off heating featuring a short on-state (~1500K for 55 ms) and a 10-times longer off-state (room temperature)," said Yao. "The high temperature provides activation energy for atom dispersion by forming strong metal-defect bonds, while the off-state critically ensures the overall stability."
Joule heating was utilized to hit the high temperature marks, and the team confirmed synthesis using in situ scanning transmission electron microscopy (STEM). This technique can be used in catalytic reactions such as methane conversion, which converts natural gas into useful chemicals such as ethylene, ethane and benzene.
“The reported shockwave method is facile, ultrafast and universal, which opens a general route for single atom manufacturing that is conventionally challenging," said Hu.
This study was a multi-institutional collaboration including the Reza Shahbazian-Yassar group at the University of Illinois, Chicago; Chao Wang's group at John Hopkin's University; Teng Li's group in the UMD Department of Mechanical Engineering; the Tianpin Wu and Jun Lu group at Argonne National Laboratory; Chongmin Wang's group at Pacific Northwest National Laboratory, and Michael Zachariah's group at UMD.
For additional information:
Nature Nanotechnology, 2019, DOI: 10.1038/s41565-019-0518-7
About the A. James Clark School of Engineering
The University of Maryland’s A. James Clark School of Engineering is a premier program, ranked among the top 20 in the world. Located just a few miles from Washington, D.C., the Clark School is at the center of a constellation of high-tech companies and federal laboratories, offering students and faculty access to unique professional opportunities.
Our broad spectrum of academic programs, including the world’s only accredited undergraduate fire protection engineering program, is complemented by a vibrant entrepreneurial ecosystem, early hands-on educational experiences, and participation in national and international competitions.
The Clark School is leading research advancements in aerospace, bioengineering, robotics, nanotechnology, disaster resilience, energy and sustainability, and cybersecurity. From the universal product code to satellite radio, SMS text messaging to the implantable insulin pump, our students, faculty, and alumni are engineering life-changing innovations for millions. Learn more at www.eng.umd.edu.