News Story
Lee, Rabin Research on Cover of Nanotechnology

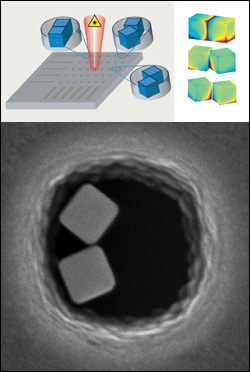
Lee and Rabin's work on the cover of Nanotechnology, showing a blossoming crystal made of silver nanoparticles.
In an earlier study, Lee, Rabin and their colleagues described how nanoscale silver cubes can enhance the effectiveness of surface-enhanced Raman scattering (SERS) by focusing and intensifying the light from a laser probe. Because the cubes are more effective in SERS when clustered, the team drove the nanocubes to self-assemble at predetermined locations, nestled in bowl-shaped pores patterned into a silicon substrate using ion-etching.
When the silver nanocubes were left unattended on the silicon, however, something unexpected and remarkable occurred: their structure changed and they merged, resulting in beautiful crystal growths resembling branches and flowers.
"Nobody anticipated a reaction that involves solid metal particles changing their shape at room temperature," says Rabin. "The effect is unique in that it only occurs at the nanoscale."
Lee and Rabin theorize that the pores in the silicon, etched by exposing it to energetic sulfur hexafluoride (SF6) ions through a patterned mask, remained reactive because they contained silicon and SF6 "shrapnel" left over from the process. When the shrapnel came into contact with silver nanoparticles, the silver was stimulated to grow into new forms. The pair also discovered that the effect is unique to nanoparticles—when the pores were exposed to a solution of silver ions, nothing happened.
"We think the reason people haven't seen this before is because you need the combination of a nanoscale material and a silicon substrate processed in this particular way," Rabin adds.
Lee and Rabin explored the reaction mechanism by modifying the reaction conditions, eventually discovering how to control and guide the growth of the crystals. Rabin thinks the discovery will lead to more awareness of the complexity of flaws in nanomanufacturing.
For More Information:
Published April 9, 2012