News Story
Liquid Crystal/Nanoparticle Mix Lays Groundwork for New Photovoltaics
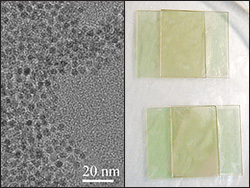
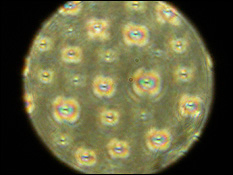
Left: The ZnO nanoparticles designed by Martínez-Miranda and Salamanca-Riba. Right: Sample electrodes made out of ZnO nanoparticles suspended in liquid crystal between two slides. Current was run through samples of containing different concentrations of nanoparticles to determine the mixture that generates the greatest flow of current.
Nanoparticles mixed with organic liquid crystals could be the cost-effective future of photovoltaic (solar powered) devices, according to a recent study published by Department of Materials Science and Engineering professors and Maryland NanoCenter members Luz J. Martínez-Miranda and Lourdes G. Salamanca-Riba. The work is the first to demonstrate a hybrid photovoltaic system made from nanoparticles and liquid crystals, and the first to prove that a specific ordering of the liquid crystals with the nanoparticles results in higher electrical currents.
The photovoltaic materials used in today's solar cells are solid state and expensive, two factors which have prevented their widespread adoption. The development of an effective liquid crystal-based photovoltaic would not only result in more affordable products, but also more design options, since the liquid crystal could be housed in flexible or custom-shaped containers, or possibly applied as a coating.
In their study, Martínez-Miranda and Salamanca-Riba show that adding nanoparticles made of zinc oxide (ZnO), a semiconductor, to an organic liquid crystal increases the flow of current.
"We found that as we increased the concentration of the nanoparticles, the mutual ordering of the liquid crystals and the nanoparticles would also increase," says Martínez-Miranda. "The liquid crystals align parallel to the electrode surface, with the nanoparticles in between them, creating more direct passages for electrical current in the system. The higher the electron flow, the greater the efficiency and the more current generated. At the optimum level of concentration we've discovered, the system we've created produces three times more current than an ordered liquid crystal could on its own."
Martínez-Miranda and Salamanca-Riba's solution does have some drawbacks, at least for now. "Solid state photovoltaics are currently about 45% more efficient," says Salamanca-Riba, "but a liquid crystal-based system would be a lot cheaper."
One of the reasons for the difference in efficiency is that ZnO, the semiconductor the pair used for their nanoparticles, conducts when exposed to blue and near ultra-violet light, which comprises only a small portion of the visible spectrum produced by the sun. The liquid crystals used absorb in the green.
"In actual production," Salamanca-Riba explains, "an effective liquid crystal photovoltaic system would require the development of nanoparticles from materials that absorb a greater range of visible light, or a combination of materials that cover the entire spectrum. We chose zinc oxide for our study because its properties are well known and we can precisely control their size, which is important in maintaining the ordering of the liquid crystal and the consistency of the current generated. It lets us create a reliable system that can help us understand the mechanics of a hybrid liquid crystal/nanoparticle photovoltaic, and act as a platform for future development."
The liquid crystal was chosen for similar reasons, Martínez-Miranda adds, even though it too would not be ideal in a production environment. "The properties of the liquid crystal we used are very well known, and how to control its alignment in a film is well established."
In the current phase of their research, the pair is replacing their vertical stacks of ZnO nanoparticles with nanowires also made of ZnO or other materials, in the hope that their long, thin shapes and ability to be fastened to a substrate will make ordering the system even more effective. Preliminary results using nanorods or nanowires of ZnO show an increase in the flow of current.
For More Information:
See: "Liquid crystal-ZnO nanoparticle photovoltaics: Role of nanoparticles in ordering the liquid crystal." L. J. Martínez-Miranda, Kaitlin M. Traister, Iriselies Meléndez-Rodríguez, and Lourdes Salamanca-Riba. Applied Physics Letters 97(1), 2010. Abstract »
Visit Professor Martínez-Miranda's homepage »
Visit Professor Salamanca-Riba's homepage »
Published March 31, 2011